
Global Contract Manufacturing
Supporting the Globalization of Pharmaceutical Companies
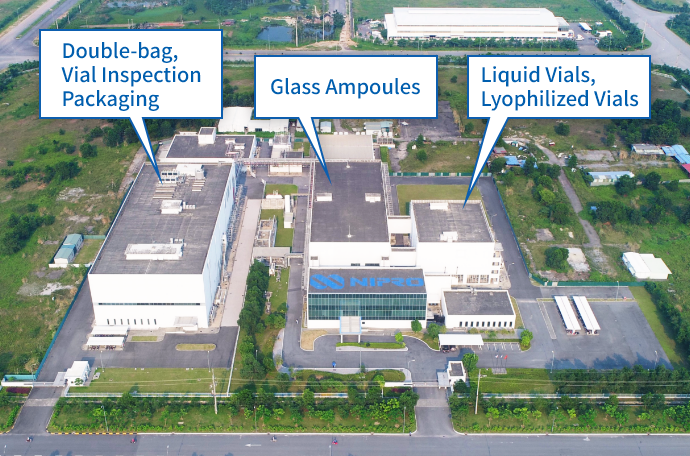
Nipro Pharma Vietnam manufactures high quality pharmaceutical products under the management and guidance of Japanese staff, and is PIC/S and WHO-GMP compliant, and has established a supply system for global markets including Japanese, U.S., European and other global markets.
Providing Japanese quality from Vietnam
Nipro Pharma Vietnam deploys production technologies that have been standardized in Japan, to provide pharmaceutical products of high quality at internationally competitive prices. In conformity with the U.S., EU and Japan GMP requirements and with PIC/S GMP, the company supports the export of products for pharmaceutical companies around the world, to the Japanese, U.S., European and global markets.

Production lines conforming with the U.S., EU, and Japan GMP requirements and with PIC/S GMP
GMP that requires prevention of contamination, prevention of human errors, and assurance of quality systems, vary by country and region. In recent years, conformity with PIC/S has also been required for the purpose of establishing globally standardized GMP. Nipro Pharma conforms with the U.S., EU, and Japan GMP requirements and with PIC/S GMP, which enables the production of contract pharmaceutical products to be exported around the world.

Best partner for global deployment
At the Kagamiishi Plant, Ise Plant, and Odate Plant we have been audited by global partners many times as a manufacturer of contract pharmaceutical products for foreign pharmaceutical companies. Based on this rich experience, we provide support for entrance into the Japanese market, including the collection of application data. We also have extensive experience in exporting pharmaceutical products consigned by Japanese pharmaceutical companies.
