
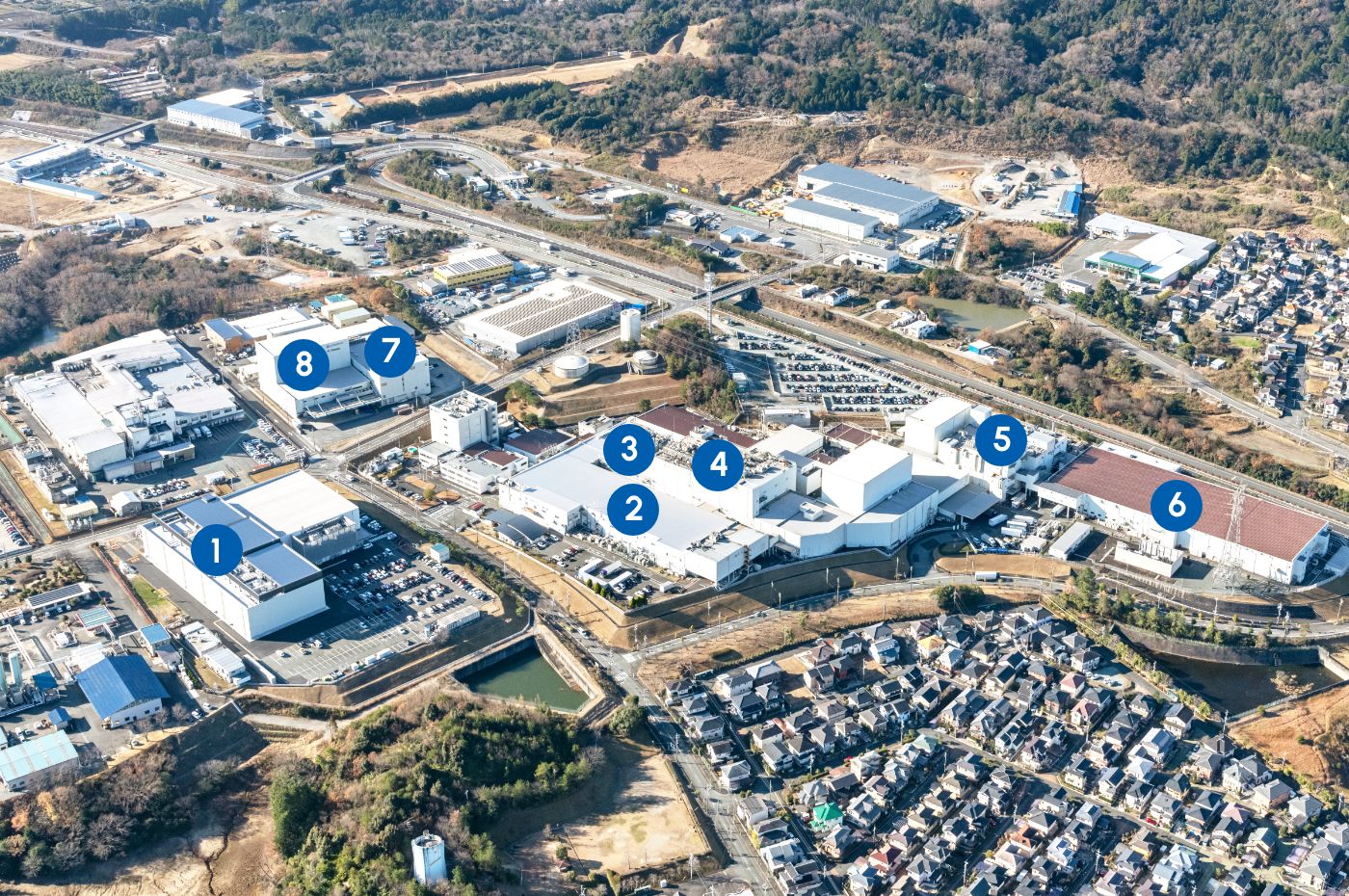
Ise Plant
Ampoule/Vial and Injectable Kit Manufacturing
State-of-the-art Syringe Product Manufacturing Bldg. Expanded

Ise Plant, which manufactures kit formulations of injectable products including syringes in addition to general ampoules and vials, has added a new pre-filled syringe (PFS®) formulation manufacturing bldg.
By consolidating know-how based on our mass production experience, we will promote low-cost production by “speeding up production tact time” and “building a labor-saving line through the introduction of new technologies such as automation. The addition of this manufacturing building to the pre-filled syringe (PFS®) production lines already in operation at the Ise Plant and Odate Plant (Odate City, Akita Pref.) will further strengthen crisis management and a stable supply system by decentralizing production bases.
Ise Plant will continue to evolve to ensure a stable supply of high-quality pharmaceutical products.
- Pre-filled syringes(PFS®)
- Glass ampoules/Half kits
- Freeze-dried vials
- Pre-filled syringes(PFS®)/
Plastic ampoules/
Fluid vials/
Freeze-dried vials - Powder dialysis agents
- Double bags(PLW®)
- Inspection and packaging
- Logistics Bldg.
Ise Plant Video Introduction

Plant for pharmaceutical inspection and packaging and the quality control bldg
At Ise plant, we started operation of the facility for pharmaceutical inspection and packaging (operated in July 2017), quality control building (operated in December 2017) and new warehouse building (operated in September 2018) to expand the capability and meet the customer needs.

Top class ampoule production line in Japan
In the Ise Plant, we produce injectables of various dosage forms. The Ise Plant is a main plant for glass and plastic ampoules, and has manufacturability for 1, 2, 5, 10 and 20 mL glass ampoules, and production lines for 5, 10 and 20 mL plastic ampoules.
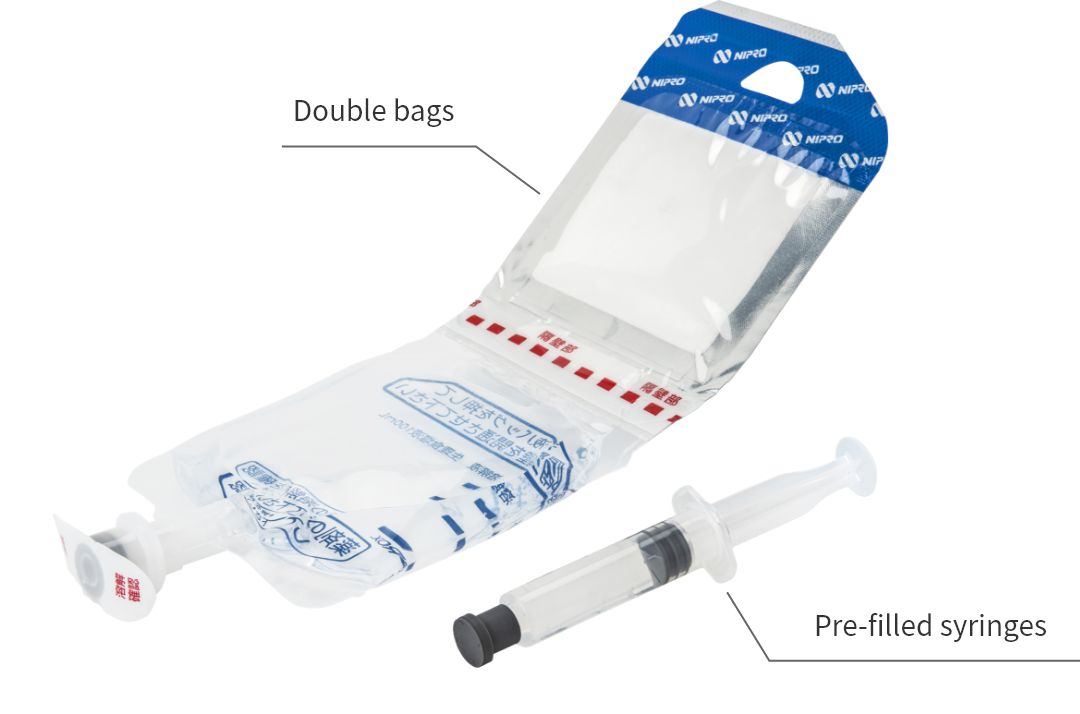
Produces injectable drugs kits in general
In this plant, we produce pre-filled syringes (syringes pre-filled with drug solution) enabling faster injection of the patient; double bags (PLW®) that combine powder drugs that require dissolution before administration with the dissolving solution, thereby enabling adjustment with aseptic manipulation; pre-mixed bags that contain a bag pre-filled with drug solution that has been adjusted to the concentration for administration; and half kits.
Quick facts about the Ise Plant
| Location |
647-240, Ureshinotengeji-Cho, Matsusaka-Shi, Mie, 515-2302, Japan |
|---|---|
| TEL |
+81-598-42-6531 |
| FAX |
+81-598-42-6533 |


