
Why Do Customers Select Nipro Pharma?
Innovation Partner
for You
Nipro Pharma is a member of the Nipro Group and is engaged in the development of safe and convenient products in all areas of pharmaceuticals, including injectable, oral, and external Preparations.
We are positioned as one of the top pharmaceutical contract manufacturers in Japan with our outstanding technological capabilities that enable us to stably produce high quality products.We will continue to contribute to society as a good partner to pharmaceutical companies in Japan and abroad, providing high quality medical care.
Nipro Group’s strengths

The Nipro Group is able to carry out the entire process from the development of pharmaceuticals, the manufacture of materials, and the production of pharmaceuticals to the sale of pharmaceuticals in a single integrated step.
Nipro Pharma manufactures pharmaceuticals and materials developed by Nipro. Nipro Pharma manufactures the pharmaceuticals and materials developed by Nipro and sells the finished products back to Nipro. The group companies share roles and responsibilities by taking advantage of their respective characteristics and strengths to efficiently manufacture and sell pharmaceutical products.
Nipro Group Cooperation


Nipro Pharma’s strengths

01.

Original
development and manufacturing technology
02.

Pharmaceutical Contract Manufacturing
Top class in Japan
03.

Supplying high-quality
products to the whole world
Original development and manufacturing technology
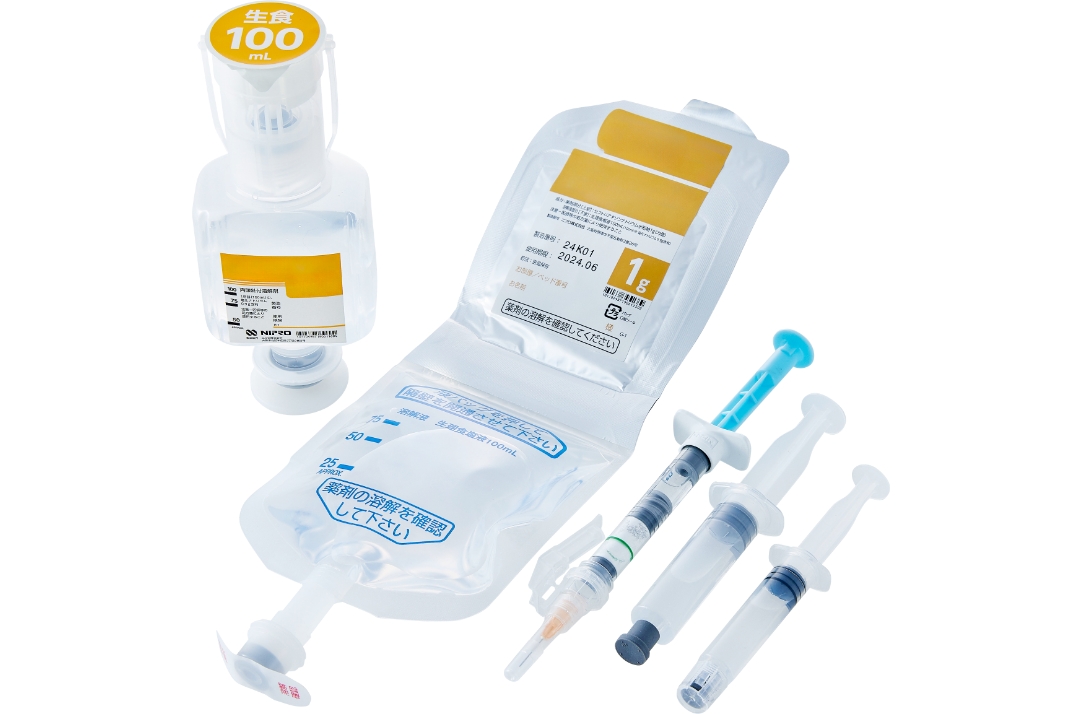
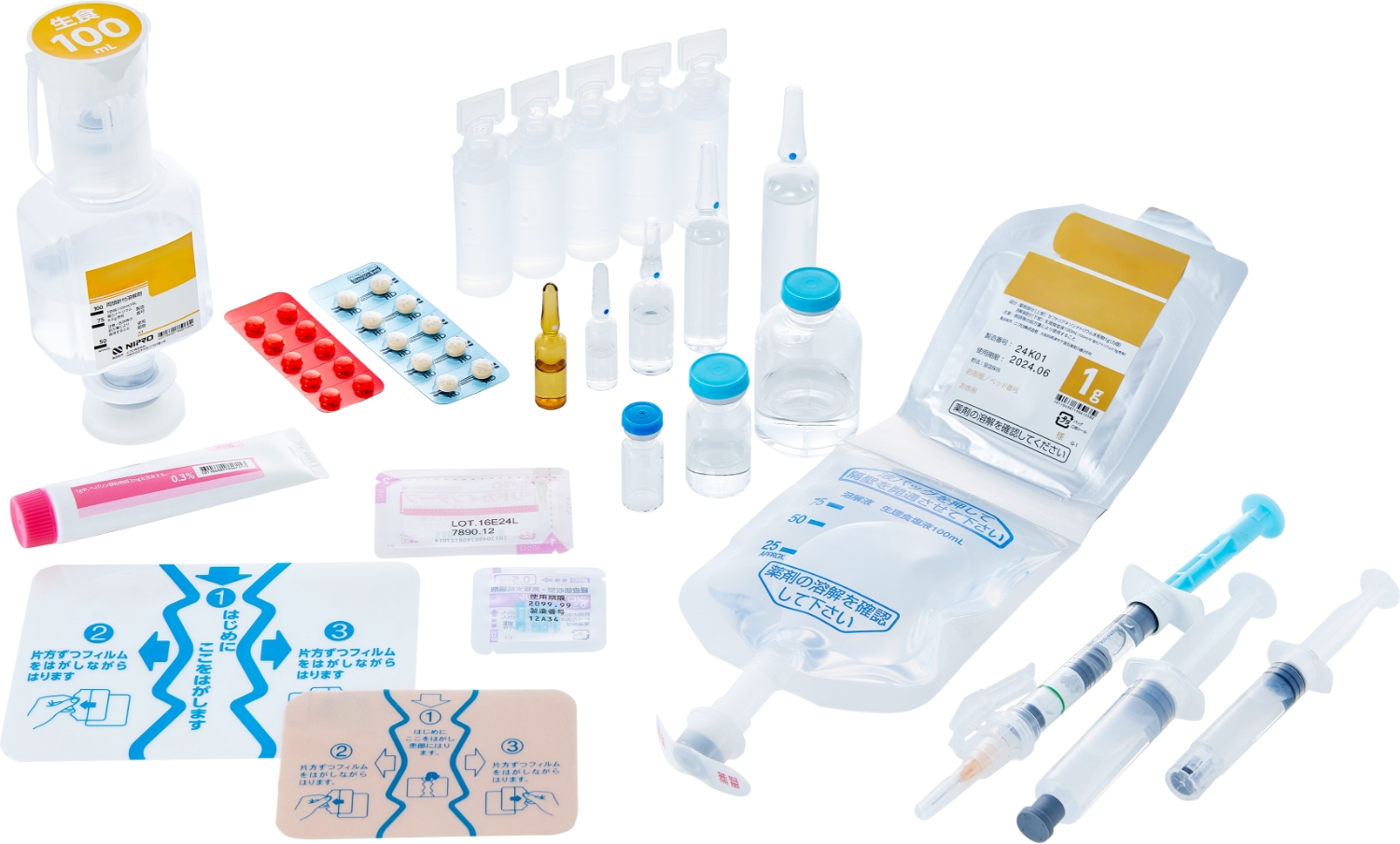
Kit formulation products guarantee a sterile environment and prevent medical errors
Nipro Pharma has profound knowledge and know-how not only about drugs but also medical devices. Its proprietary kit formulation products include pre-filled syringes and double chamber bags (PLW®). These kit formulation products guarantee a sterile environment, prevent medical error, and contribute to alleviating the workload in medical institutions.
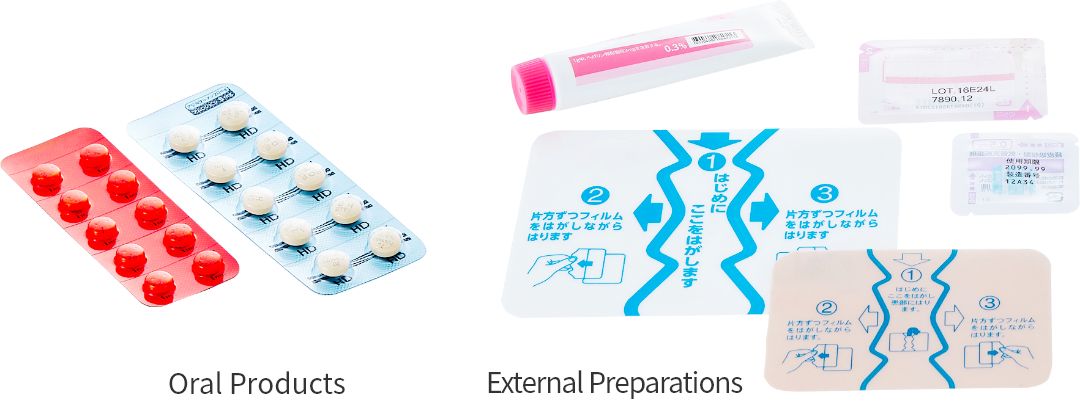
Oral Products and external preparations that feature convenience for customers
We have a production technology for oral products, which easily dissolve in the mouth, in the category of oral products, and have developed tablet packaging which has a blank space for writing a date of taking the drug to prevent missed taking. For external preparations, we have developed Transdermal Therapeutic System (TTS), where the drug is delivered through the skin to the systemic blood circulation, as well as plasters that can be easily detached and re-attached. We contribute to improve the QOL of customers by our formulation technology, for example, switching from oral products to external preparations.
Pharmaceutical Contract Manufacturing Top class in Japan

From production of investigational products to commercial production From development to production
We cover all dosage forms from injectables to oral products and external preparations. We have the production capacity to cover all steps from the stage of investigational products to post-marketing mass production, and to satisfy all requests from development to production and to development of containers. We also have state-of-the-art containment facilities that can produce highly active agents, such as anticancer drugs, steroids and hormone drugs, and are capable of producing contract biomedicines using drug substances supplied by customers.

CDMO experience
Nipro Pharma has a top-class domestic track record in contract manufacturing of injectable, oral, and external preparations. In addition to the production of a wide variety of dosage forms, we are equipped with a system that can accommodate a wide range of production scales. As a member of the Nipro Group, Nipro Pharma is highly regarded for its strength in providing a one-stop solution for the diverse needs of pharmaceutical companies, from development to manufacturing.
Supplying high-quality products to the whole world

Best partner to expand in global markets
Kagamiishi Plant, which manufactures oral products, and Ise and Odate Plants, which manufacture injectables, have undergone numerous global audits to produce pharmaceutical products for overseas pharmaceutical companies. As a global CDMO, we supply our products to more than 30 countries. We support both companies which want to expand their business oversea and to enter the Japanese market.

Enables supply from Vietnam to all global markets
Nipro Pharma Vietnam plans to start with injectables and expand the production line to oral and external preparations in the future. Nipro Pharma Vietnam proactively secures excellent local human resources and manufactures pharmaceutical products of reliable quality in collaboration with Japanese staff. We are also compliant with the Trilateral GMP and PIC/S GMP, and have established a supply system for the global market, including Japan, U.S., and Europe.
About quality

Quality Policy
01.
To improve the quality of life (QOL) of patients around the world, we will continue to supply reliable and secure pharmaceutical products with our highly original technologies and outstanding manufacturing capabilities.
02.
The management team will take the lead in the idea of prioritizing quality, and each staff member will work for continuous improvement of quality with a sense of ownership.
03.
We will not only comply with laws and regulations, but also maintain responsible corporate activities through honest and diligent efforts by all employees.
Certification

BCP
We have established BCP manuals for each of our plants, and are promoting initiatives to ensure that even in the event of a large-scale disaster, we can promptly resume production of and provide a stable supply of the government's stabilized pharmaceuticals and other products.
ISO
We have acquired ISO 14001 and Eco Action 21 certification for our environmental management system and ISO 45001 certification for our health and safety management system on a plant-by-plant basis, and are implementing management in accordance with these systems.
FDA Certification
Kagamiishi Plant, which manufactures solid dosage forms, and Odate Plant, which manufactures injectables (pre-filled syringe formulations and high potency formulations), have received FDA certification for their manufacturing. There are only a few contract pharmaceutical manufacturing organizations (CDMOs) in Japan that are FDA-certified, and FDA certification for sterile products is even rarer.
